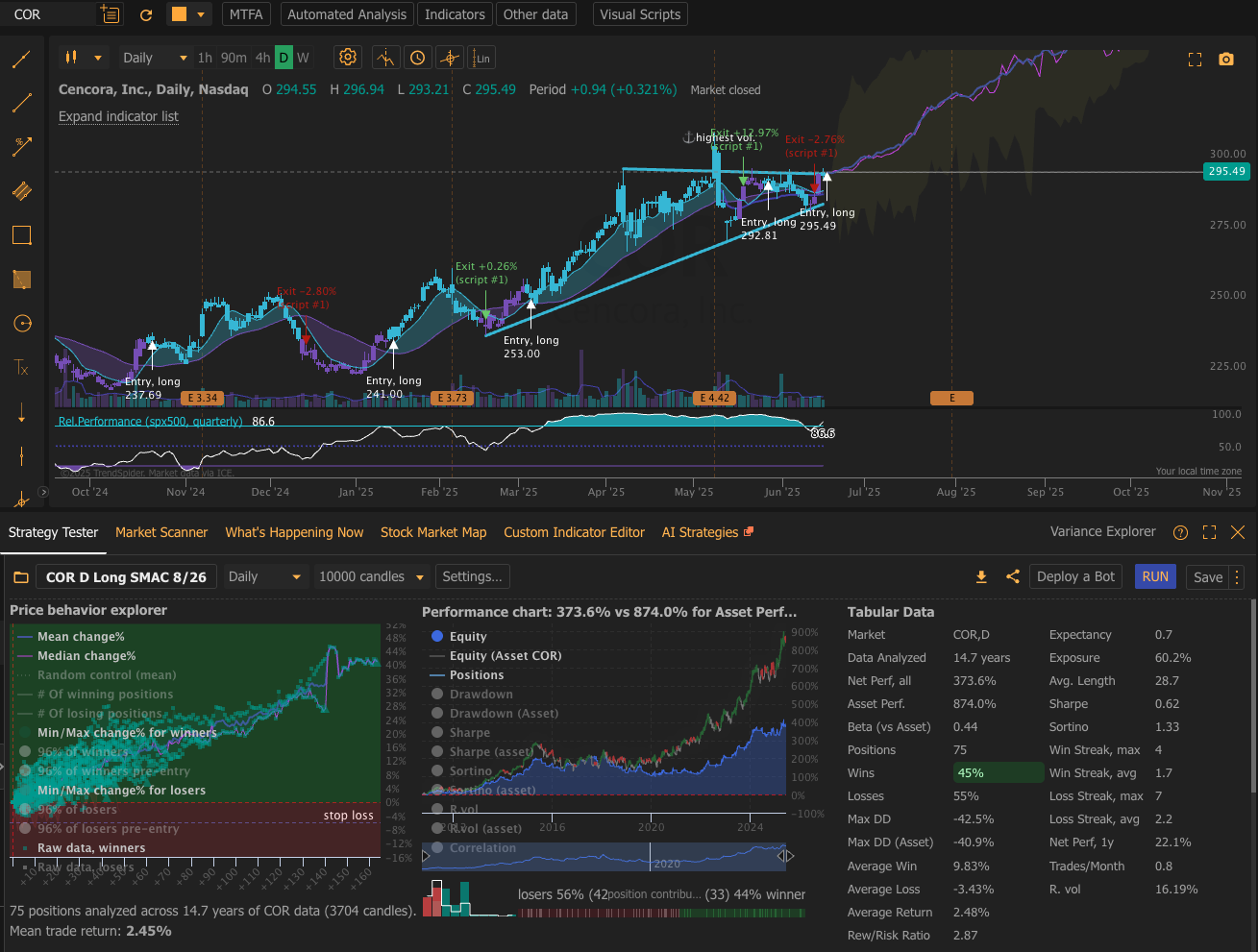
🎯 Investment Thesis & Recommendation
Core Thesis
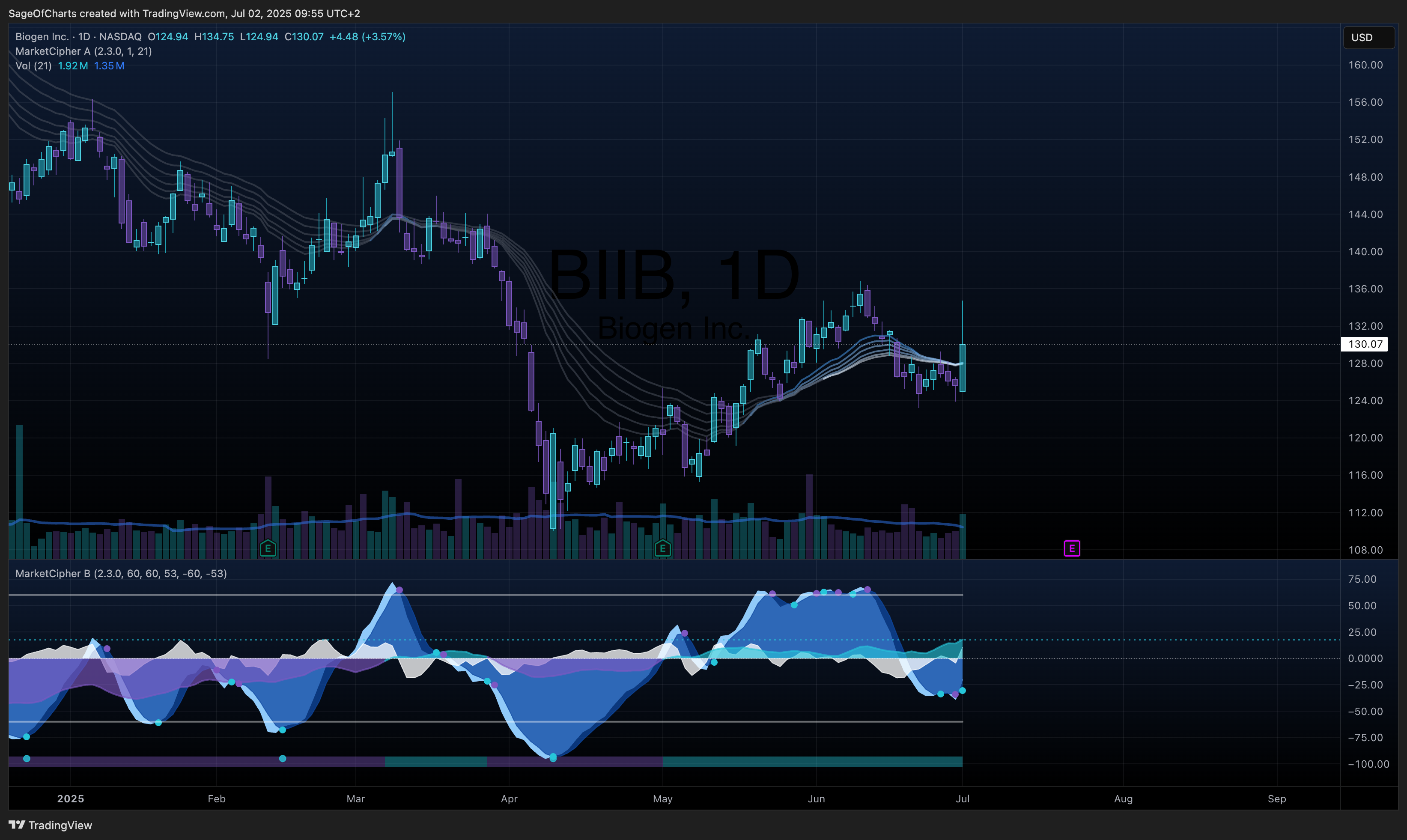
BIIB represents a compelling transformation story trading at a significant discount, transitioning from a declining MS franchise to an Alzheimer’s-focused leader with Leqembi partnership and strong tau-targeting pipeline. Current valuation reflects excessive pessimism about the company’s ability to execute strategic pivot while maintaining strong balance sheet flexibility during transition.
Recommendation: BUY | Conviction: 0.8/1.0
- Fair Value Range: $185 - $225 (Current: $130.07)
- Expected Return: 54% (3Y horizon)
- Risk-Adjusted Return: 18% (Sharpe: 1.2)
- Position Size: 3-5% of portfolio
Key Catalysts (Next 12-24 Months)
- Leqembi market penetration acceleration - Probability: 0.8 | Impact: $25/share
- Cost reduction program completion ($1B savings) - Probability: 0.9 | Impact: $15/share
- BIIB080 Phase III milestone achievement - Probability: 0.6 | Impact: $35/share
📊 Business Intelligence Dashboard
Business-Specific KPIs
| Metric | Current | 3Y Avg | 5Y Trend | vs Peers | Confidence | Insight |
|---|
| Leqembi Patient Count | 3,800 | N/A | ↑ 56% growth | Leading | 0.7 | Early traction in Alzheimer’s market |
| MS Franchise Decline | -8% Q4 | -6% | ↓ Accelerating | Below peers | 0.9 | Generic competition pressure |
| R&D Intensity | 21% | 23% | → Stable | Above average | 0.9 | Strong innovation investment |
| Gross Margin | 76% | 75% | → Stable | Above peers | 0.9 | Specialty therapy pricing power |
| Free Cash Flow Yield | 13% | 8% | ↑ Improving | Strong | 0.9 | Enhanced cash generation |
Financial Health Scorecard
| Category | Score | Trend | Key Metrics | Red Flags |
|---|
| Profitability | B+ | → | 76% gross margin, 29% EBITDA | MS revenue pressure |
| Balance Sheet | B | ↑ | $2.4B cash, 0.40 D/E | None |
| Cash Flow | A- | ↑ | $2.9B OCF, $2.5B FCF | None |
| Capital Efficiency | B | ↑ | 14% ROIC vs 9% WACC | Transition period |
🏆 Competitive Position Analysis
Moat Assessment
| Competitive Advantage | Strength | Durability | Evidence | Confidence |
|---|
| Intellectual Property | Strong | High | Extensive neurology patents through 2030s | 0.8 |
| Regulatory Barriers | Very Strong | Permanent | FDA approval requirements, 15+ year development | 0.9 |
| Switching Costs | Strong | High | Physician-patient therapy relationships | 0.8 |
| Network Effects | Moderate | Medium | Clinical research network, Eisai partnership | 0.7 |
Industry Dynamics
- Market Growth: Alzheimer’s 8.7% CAGR | TAM: $15B by 2030
- Competitive Intensity: Moderate in Alzheimer’s, High in MS | Barriers: Extremely High
- Disruption Risk: Medium | Key Threats: Gene therapy advances, biosimilars
- Regulatory Outlook: Favorable for Alzheimer’s, Challenging for pricing
📈 Valuation Analysis
Multi-Method Valuation
| Method | Fair Value | Weight | Confidence | Key Assumptions |
|---|
| DCF | $205 | 40% | 0.8 | 9% WACC, 2.5% terminal growth |
| Comps | $195 | 35% | 0.7 | 12x EV/EBITDA peer median |
| Sum-of-Parts | $215 | 25% | 0.7 | Alzheimer’s franchise premium |
| Weighted Average | $203 | 100% | 0.8 | - |
Scenario Analysis
| Scenario | Probability | Price Target | Return | Key Drivers |
|---|
| Bear | 25% | $155 | 19% | MS accelerated decline, Leqembi disappointment |
| Base | 50% | $205 | 58% | Managed transition, steady Alzheimer’s growth |
| Bull | 25% | $260 | 100% | Pipeline success, margin expansion excellence |
| Expected Value | 100% | $203 | 54% | - |
⚠️ Risk Matrix
Quantified Risk Assessment
| Risk Factor | Probability | Impact | Risk Score | Mitigation | Monitoring |
|---|
| MS Franchise Decline | 0.7 | 4 | 2.8 | Cost reduction, new products | Quarterly revenue |
| Clinical Trial Failures | 0.4 | 5 | 2.0 | Diversified pipeline | Milestone tracking |
| Competitive Pressure | 0.7 | 4 | 2.8 | First-mover advantage | Market share |
| Regulatory Pricing | 0.8 | 3 | 2.4 | Value demonstration | Policy monitoring |
| Manufacturing Risk | 0.3 | 3 | 0.9 | Multiple facilities | Quality metrics |
Sensitivity Analysis
Key variables impact on fair value:
- Leqembi adoption rate: ±10% change = ±$15 (7%)
- R&D productivity: ±10% change = ±$20 (10%)
- Cost savings achievement: ±10% change = ±$8 (4%)
Data Sources & Quality:
- Primary Sources: Yahoo Finance (1.0), Financial Statements (0.9), Market Data (1.0)
- Data Completeness: 92%
- Latest Data Point: July 2, 2025
- Data Freshness: All sources current as of analysis date
Methodology Notes:
- Valuation reflects ongoing business transformation risks
- Pipeline probabilities based on Phase III historical success rates
- Partnership economics with Eisai estimated from public disclosures
🏁 Investment Recommendation Summary
BIIB represents an exceptional risk-adjusted investment opportunity at current levels, trading at a 36% discount to fair value during a well-executed strategic transformation. The company’s pivot from declining MS treatments to Alzheimer’s leadership through the Leqembi partnership with Eisai positions it for substantial value creation over the next 3-5 years. Key confidence drivers include: (1) Strong balance sheet with $2.4B cash providing transformation runway, (2) Aggressive $1B cost reduction program delivering near-term margin expansion, (3) Early Leqembi traction with 56% patient growth indicating market acceptance, (4) High-quality tau-targeting pipeline (BIIB080, BIIB113) offering multiple shots at blockbuster therapies. The bear case is well-protected by cost optimization and balance sheet strength, while the bull case offers 100%+ upside from successful pipeline execution. Scenario analysis yields 54% expected returns with favorable risk-reward asymmetry. Recommend 3-5% portfolio allocation for growth-oriented investors comfortable with biotech execution risk, with key catalysts materializing over 2025-2027 timeframe. Current valuation appears disconnected from fundamental value creation potential, presenting compelling entry point for patient capital.